On March 14, 2012, the Academy presented the Francis Amory Prize to Patrick C. Walsh, M.D., a renowned urologist who pioneered work in the understanding and treatment of prostate cancer. (The prize citation was printed in the Spring 2012 issue of the Bulletin.) Following the prize ceremony, Dr. Walsh participated in the Francis Amory Prize Symposium on advances in reproductive biology and medicine. The symposium also included presentations by David C. Page (Whitehead Institute; MIT) and Patricia K. Donahoe (Massachusetts General Hospital; Harvard Medical School). The prize ceremony and discussion served as the Academy’s 1983rd Stated Meeting. The following is an edited transcript of the symposium.
The Y Chromosome

David C. Page
David C. Page is Director of the Whitehead Institute; Professor of Biology at the Massachusetts Institute of Technology; and a Howard Hughes Medical Institute Investigator. He was elected a Fellow of the American Academy in 2011.
As the first speaker of this Francis Amory Prize Symposium, I would like to offer some comic relief. Before we get to the substantive presentations by Drs. Donahoe and Walsh, I will bring you late-breaking news from the Y chromosome. I have spent the better part of my career defending the honor of the Y chromosome in the face of innumerable insults to its character and future prospects. Let me now address these questions in the context of discoveries that my colleagues and I published this month.
You may have heard some nasty rumors that the Y chromosome is slowly withering away, and that it is on course to perish in a few million years. For the past decade or so, these rumors have appeared in leading scientific journals, and they are occasionally resurrected at conferences. Where did these ideas about the disappearing Y chromosome come from, and what is the truth about the fate of the Y chromosome? To answer these questions, I will give you a crash course in what we now understand to be the origins of not only the Y chromosome but also the X chromosome. Three hundred million years ago, when we were reptiles (the old days you talk about at Thanksgiving and other family gatherings), we existed as males and females, but we had no sex chromosomes. We had only what in our laboratory we call “ordinary” chromosomes. Others call them autosomes. But about three hundred million years ago, a perfectly ordinary, matched pair of chromosomes began a journey.
These ordinary chromosomes had swapped genes back and forth every time eggs and sperm were made–that is, until one member of this unsuspecting pair acquired a mutation that would give rise to the sex-determining gene, SRY, on the Y chromosome. Over time, first in the immediate vicinity of SRY and then over a larger region, sexual recombination– that swapping of genes with a partner chromosome– stopped. It turns out that swapping genes in the making of eggs and sperm is very good for the health and well-being of genes over long expanses of time; within the region that had stopped swapping with the X, the Y chromosome started losing genes and began to shrink (Figure 1). This process eventually took over most of the Y chromosome, so that while today’s X chromosome retains the gene content of the ancestral autosomes, the Y chromosome is a mere vestige of its former glory. If you extrapolate into the future, it does not look good for the Y chromosome.
Figure 1
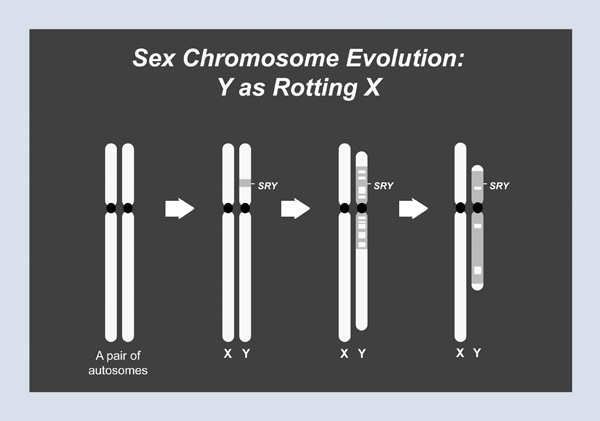
This doomsday scenario did not escape the attention of researchers in the field. In fact, in an editorial published in Nature a decade ago, somewhat grandly titled “The Future of Sex,” my colleagues John Aitken and Jenny Graves recount what I have just told you: that the Y chromosome is particularly vulnerable across evolutionary time because it does not have a matching partner with which it can swap and retrieve lost genetic information. They went on to state that the original Y chromosome, that ancestral autosome, carried “around 1,500 genes; but during the ensuing three hundred million years, all but around 50 were inactivated or lost.” And then came the devastating analytic part of this editorial, which took these two data points (300 million years ago, 1,500 genes; today, 50 genes), drew a straight line through them, and reached the following conclusion: “at the present rate of decay, the Y chromosome will self-destruct in around ten million years.”
I was not the first member of my laboratory to read this editorial. It was one of my graduate students who came running into my office, tears streaming down his face. We held an emergency lab meeting in which we resolved to pick up the pace of our research. So let me tell you briefly what we have learned in the ensuing decade.
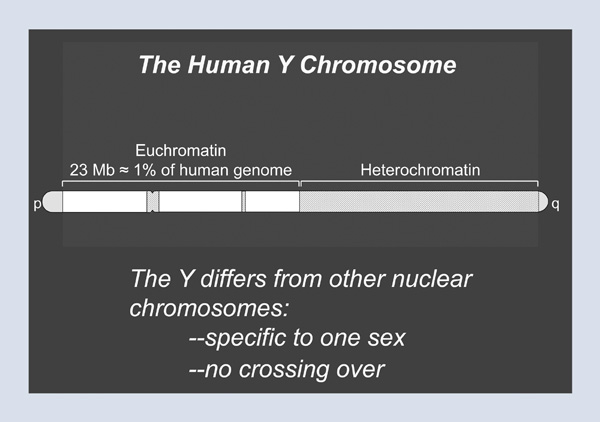
Figure 2 shows a schematic of the human Y chromosome as we understand it. At either end of the chromosome are areas where the X and the Y chromosomes routinely swap genetic information. These areas are called the pseudoautosomal regions. In between lies the strictly male-specific part of the Y chromosome, which is distinguished from all other nuclear chromosomes by two features. First, it is specific to one sex; all other chromosomes have traveled through both males and females. Second, it does not participate in crossing-over or swapping with a homologue. In fact, much of the long arm of the Y chromosome is so-called heterochromatic (see Figure 2). That is, it has a very simple, monotonous sequence composition and is such a dense jungle of repeats that, sadly, no molecular biologist has entered the heterochromatic region and returned alive. So tonight, we will instead focus on the euchromatic part of the Y chromosome, which makes up a bit less than 1 percent of the human genome.
Figure 2
I will now summarize all that has been learned in several laboratories, including my own, over the last decade. In doing so, I will contrast the slurs of the past with the understanding of the present.
First, the notion that the Y chromosome is a genetic wasteland: we now understand that the Y chromosome carries about seventy-six protein-coating genes, many of which have specialized roles in the production of sperm.
Second, the image of the Y chromosome as merely a rotting copy of an ancient autosome: we understand today that the Y chromosome still carries some of the genes from that ancestral autosome. However it has also imported other genes from elsewhere in the genome, especially during primate evolution, and has even amplified some of them.
Third, the idea that the Y chromosome is full of junky repeats: we have learned that the Y chromosome actually carries palindromes – that is, almost perfectly symmetrical structures of unbelievable size, scale, and precision.
Fourth, the notion that all of the Y chromosome’s genes are disintegrating: today we understand that special mechanisms operating within the palindromes contribute to the evolutionary longevity of gene pairs located there.
Fifth, the theory that the Y is headed for extinction: it is now apparent that even the single-copy genes of the Y, those not benefiting from the palindrome mechanism, are doing quite well.
Finally, in an age of translational research, the ultimate slur is that the Y chromosome is of no medical significance. We have learned in the last ten or fifteen years that the Y chromosome holds many of the answers to male infertility (spermatogenic failure), and it may hold important answers in the case of testis cancer and Turner syndrome as well.
In closing, I will turn to the work that we published just this month. It is a tale of three primates, and it directly addresses the claim that the Y chromosome will go extinct within ten million years. I will tell the story through a comparison of the Y chromosomes of three primates. The star of this show is the rhesus monkey, whose Y chromosome we will compare with the Y chromosome of a chimpanzee named Clint as well as that of none other than tonight’s honoree, Dr. Patrick Walsh. (This is an appropriate time to disclose publicly the fact that the Y chromosome we sequenced eight or nine years ago was that of Dr. Walsh. We have not worked out all the issues of informed consent, but that will be dealt with later this evening.)
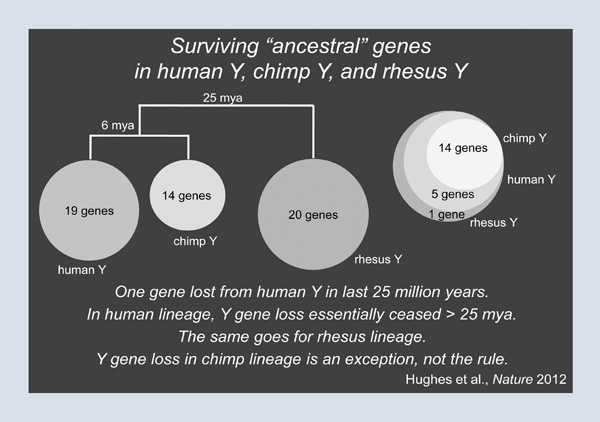
What does comparing Patrick’s Y chromosome with those of Clint and the rhesus monkey tell us about the future and the fate of the Y? We are going to look at a set of genes that we called the ancestral genes, that is, the genes of the Y’s autosomal ancestry. On Patrick’s Y chromosome, there are nineteen such genes. Clint the chimp has only fourteen such genes, and these fourteen form a nested subset of Patrick’s nineteen (Figure 3). This finding suggests that no Y-chromosomal genes have been lost in the human lineage since the split between human and chimpanzee six million years ago. But did the Y chromosome of the human-chimpanzee progenitor carry nineteen ancestral genes? To answer this question, we turn to the rhesus monkey, and we switch from a six million-year comparison to a twenty-five million-year comparison. Here, we find that the rhesus monkey has twenty ancestral genes, and that Patrick’s nineteen are completely nested within those twenty (Figure 4). In other words, Patrick’s Y chromosome carries almost exactly the same set of genes as the Y chromosome of the rhesus; this implies that in the lineage leading to Patrick’s Y chromosome and to all other Y chromosomes in this room, one gene has been lost in the last twenty-five million years. In other words, in the human lineage, gene loss on the Y chromosome essentially ceased more than twenty-five million years ago. The same goes for the rhesus lineage. As it turns out, the chimp is the exception, not the rule. So I want all the men in the audience to come away from this lecture having breathed a huge sigh of relief.
Figure 3
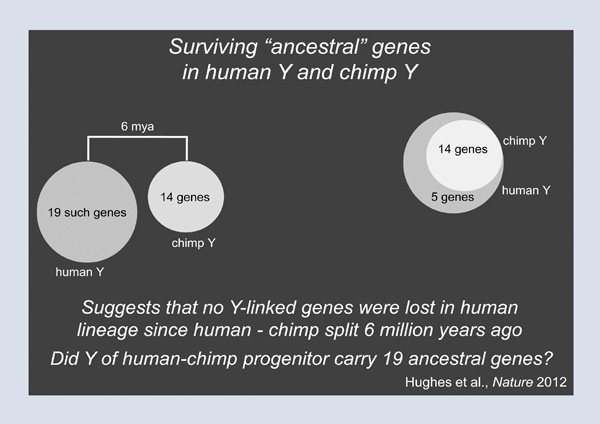
Figure 4
Developmental Reproductive Biology: Advances Impacting Disorders of Sexual Development and Ovarian Cancer

Patricia K. Donahoe
Patricia K. Donahoe is the Marshall K. Bartlett Professor of Surgery at Harvard Medical School and Director of the Pediatric Surgical Research Laboratories and Chief Emerita of Pediatric Surgical Services at Massachusetts General Hospital. She has been a Fellow of the American Academy since 1987.
Developmental reproductive biology has had a substantial influence on our clinical care of children with disorders of sexual differentiation. The molecular factors so important in normal sexual differentiation have also influenced the way we care for patients with ovarian cancer.
Sexual differentiation is an amazing cascade of events, each of which must be correct for proper sexual differentiation to occur. One must first have the right chromosomal endowment. Once the gonads under the influence of this chromosomal endowment have properly differentiated, they must make the right hormones. Then other tissues must respond appropriately to those hormones in order for an appropriate phenotypic male or female to eventuate. Sexual differentiation, which occurs midway through mammalian development after the primitive streak forms and before gastrulation, is driven by the intermediate mesoderm, which is located between the ectoderm and the endoderm in the early embryo. At that time, a number of transcription factors come into play, allowing further differentiation of the intermediate mesoderm into the urogenital ridge.
The urogenital ridge is posteriorly and dorsally placed in the embryo and is sexually indifferent until the gonad has declared itself as a testis or an ovary (Figure 1); both male and the female reproductive tracts are present and in close approximation, at this time, as are the subjacent kidneys and superjacent adrenals. The urogenital ridge is then exposed to the transcriptional master switch, the sex-differentiation gene SRY, located on the sex-determining region of the Y chromosome. The only function ascribed to this tiny gene is to bend DNA. One wonders how such a potent master switch can be such a simple molecule. As a transcription factor, it is responsible for the differentiation of the somatic components of the gonad.
Figure 1
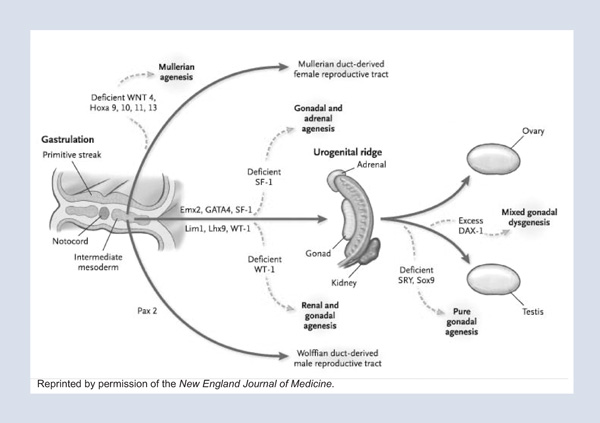
Before somatic differentiation of the gonad occurs, a small number of cells within the epiblast differentiate to become germ cells. These germ cells, which perpetuate pluripotency and propagate the gene pool, undergo an extensive migration from the epiblast, along the primitive streak, and to the hindgut from which they eventually populate the differentiating gonad in the urogenital ridge.
One of Dr. Page’s great contributions, in addition to defining the sex-determining region of the Y chromosome, was the discovery of the mechanisms contributing to meiosis inhibition, which differentiates male and female gonads, as female germ cells enter meiosis just before birth, while the male gonad produces a “meiosis inhibiting factor” which prevents meiosis until puberty, many years later.
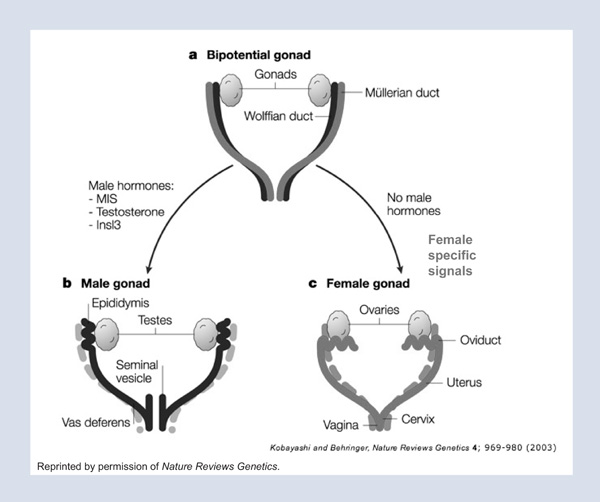
If the gonad differentiates as a testis under the influence of SRY, it produces two products. One is testosterone, which is further modified by five alpha reductase, one of the genes that Patrick Walsh discovered with his early mentor, Jean Wilson. This reduced form of testosterone acts on the external genitalia, resulting in male differentiation. Under the influence of dihydrotestosterone (dHT), the genital tubercle becomes a phallus, and the genital folds become the scrotal organs housing the descended gonad. In the face of syndromes resulting in testosterone deficiency, this differentiation cannot be completed, resulting in smaller structures. There are also syndromes in which the adrenal produces an excess of testosterone, which, in an XX female, will influence the development of the external genitalia to a more male phenotype; in these patients the clitoris is enlarged and the labia are accentuated as scrotal folds. We undertake surgery in xx patients who were exposed to excess endogenous testosterone when they are very young to reduce both the clitoris and the labia and to bring the vagina down to the perineum so that after surgery, the 46XX female patient has a small normal appearing female clitoris, petite labial folds, and an exteriorized vagina.
The other protein made by the differentiated testis is Müllerian Inhibiting Substance (MIS), with which I and my colleagues have had a lifelong interest. In the male, this potent hormone is responsible for complete regression of the Müllerian duct that would otherwise go on to form the Fallopian tubes, the uterus, and the vagina of the female (Figure 2).We hypothesized that if this protein can cause complete regression of the Müllerian duct, then it might be a potential therapeutic against tumors of Müllerian duct origin (that is, cancers of the Fallopian tube, Dr. Robert Scully, a renowned reproductive pathologist at the Massachusetts General Hospital, suggested, “Look also at ovarian cancer because ovarian cancers recapitulate the Müllerian duct of the embryo.” In validating this prediction, over the last few decades, we have purified MIS and, with colleagues at Biogen, have cloned the gene for MIS and scaled up its production. We later (along with many other labs studying this subject) cloned its receptors and studied its signal transduction pathway. Meanwhile we defined its clinical use in ovarian, breast, prostate, cervical, and endometrial cancers. To scale up production of MIS, we collaborated with Ipsen to develop MIS for potential clinical trials against ovarian cancer. This scaled human recombinant MIS inhibited ovarian cancers in vitro, as well as in animals treated in vivo for long periods.1
Figure 2
More recently, we began to consider the possibility that ovarian cancer is a stem cell disease. Could we isolate its stem cells and determine their responsiveness to MIS? This hypothesis was raised since gynecologists-oncologists can be seemingly 100 percent successful in eradicating ovarian cancer when surgery is combined with chemotherapeutic agents, but in 70 percent of patients, the tumors return and are lethal within one or two years. We looked for markers that would allow us to enrich the stem cell population, screened 130, and selected those markers that would be gentle to the cells when used for separation and compatible with flow cytometry. The marker panel chosen allowed enrichment of a select stem cell-like population of tumor cells, validated by their ability to form colonies and migrate in vitro, and, when injected in mice after limiting dilution, to show early tumor formation.
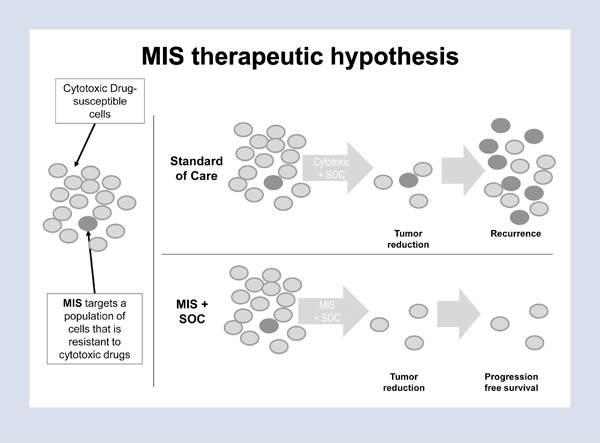
As we expected, these stem cell populations responded to MIS as well as to a small molecule MIS mimetic previously discovered in the laboratory.2 But to our surprise, when we exposed this select population to the chemotherapeutic agents that we currently use to treat ovarian cancer, we stimulated that population. One result showed a twentyfold growth of those select tumor cells treated with doxorubicin and a four to fivefold growth of those treated with cisplatin.3 Since this could represent a ratio change because the chemotherapeutic agents kill off so many other cells, we then confirmed by using colony formation assays that the same phenomenon occurred quantitatively.
The implications of this finding, recently published in the Proceedings of the National Academy of Sciences, is that ovarian cancers are initially heterogeneous with a population that not only does not respond to chemotherapeutic agents but is actually stimulated by these clinically used agents.4 Therefore, our therapeutic strategies and care of our patients must include individualized treatment of the stem cell population as well as the bulk of the tumor. Figure 3 illustrates a suggested shift in the future care of patients with ovarian cancer. We should address not only the bulk of the tumor, which responds very well to standard chemotherapeutic agents, but also the stem cell population that completely evades and is actually stimulated by current chemotherapeutic agents, but responds to MIS. We hope that, in the future, both of these populations will be treated effectively and that the treatments will be selected in a patient-specific manner.
Figure 3
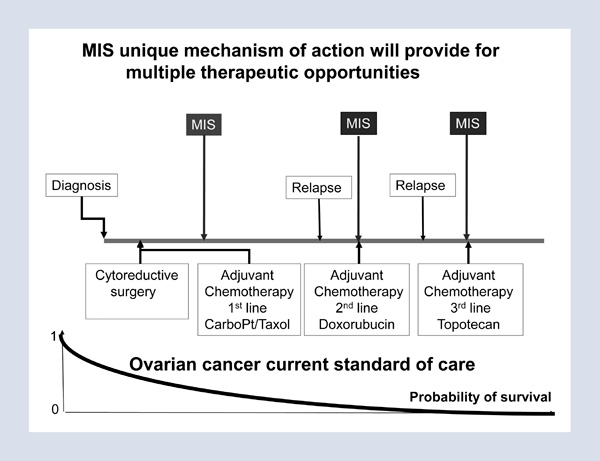
We have scaled up production of MIS and hope to take it to commercial development for treatment of patients. Toward that end, we formed a company, Mulleris Therapeutics Incorporated, and are working to continue the scale-up that we began successfully with Ipsen/Biomeasure in 2008. Future ovarian cancer therapy should continue to provide the standard of care with cryoreductive surgery and platinum-based and Taxol therapies; however, instead of subsequently treating women only when they manifest a recurrence of the tumor, we recommend treating the stem cell population so that recurrence of the tumor is averted (Figure 4).
I would like to thank my colleagues, particularly David MacLaughlin and Jose Teixeira, as none of this work is ever done in isolation. Advances in our understanding of reproductive biology have had an impact on the surgical and endocrinologic management of children born with abnormalities or disorders of sexual differentiation. Some of the discoveries of developmental biology made in the care of these children may also benefit those who face the possibility of reproductive cancers, such as ovarian cancer.
Figure 4
ENDNOTES
The Impact of Anatomic Discoveries on Prostate Cancer Surgery

Patrick C. Walsh
Patrick C. Walsh, the 2012 Francis Amory Prize recipient, is University Distinguished Service Professor of Urology at Johns Hopkins Medical Institutions.
For tonight’s last talk, I am going to tell a story. The story is about the impact of anatomic discoveries on prostate cancer surgery, and it begins with Hugh Hampton Young, the founder of modern urology, who performed the first radical prostatectomy via the perineal approach at Johns Hopkins in 1904. Young was awarded the Amory Prize in 1940. In 1947, an Irish surgeon named Terence Millin developed the radical retropubic approach, which is similar to the transabdominal approach used today, and he received the Amory Prize in 1954.
But by 1970, radical prostatectomies were rarely performed, despite their effective control of cancer, because of major side effects. There was major bleeding, often life threatening. One hundred percent of men who underwent the procedure were impotent, and 10 to 25 percent were completely incontinent. Patients and their physicians thought that the treatment was worse than the disease. When I arrived at Johns Hopkins in 1974, I was surprised to realize that even at the institution where the operation originated, it was rarely performed. At that time, I wondered why the side effects occurred and if they could be prevented. I do not believe that I would have ever made the contributions that I have made to the field of urology had I not been at Hopkins.
Hopkins has been a wonderful place to be. Though it goes unspoken, there is an expectation that your major job is discovery. On my first day, I went to lunch and sat next to the distinguished neuroscientist Vernon Mountcastle, who is a member of the American Academy. I introduced myself, saying, “I’m the new urologist.” And although I expected him to respond with the same line I’d heard over and over again–“oh, you’re the new plumber”–I heard something different. He said, “What’s your field of research?”
To make an important discovery, you need an important problem to work on, and I decided that I would try to find out why the side effects of the prostatectomy occurred. I learned very quickly that these side effects occurred because we did not understand the anatomy around the prostate. Bleeding occurred because the anatomy of the major veins responsible for the bleeding had not been charted. Impotence occurred because the location of the innervation to the corpora cavernosa was not known. And incontinence occurred because our understanding of the sphincter responsible for passive urinary control was incorrect. Why? It was because all of this anatomy had been studied in the adult cadaver. In adults, the prostate is shrouded by dense fascia, which conceals the surrounding anatomy. In the postmortem state, the abdominal viscera compress the pelvic organs into a thick pancake of tissue, and formalin fixatives dissolve the fatty planes, making the identification of anatomic structures impossible. The solution was to use the operating room as an anatomy laboratory and to perform fetal dissections. All of this is chronicled in an article published on the twenty-fifth anniversary of the first nerve-sparing radical prostatectomy.1
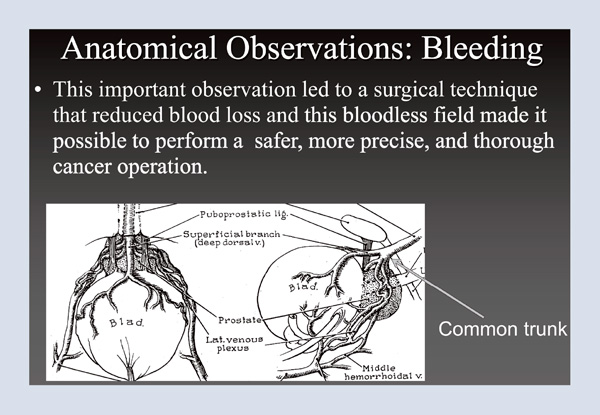
The first thing I tackled was bleeding. Using the operating room as an anatomy laboratory, I identified a common trunk over the urethra (Figure 1), hidden underneath a shroud of tissue. Merrill Sosman, the great radiologist at Brigham and Women’s Hospital in Boston, had the expression, “You only see what you look for and you only look for what you know.” Here, I was looking for something I didn’t know. Identifying the common trunk was an important observation because it led to a surgical technique that reduced blood loss; in turn, having a bloodless field made it possible to perform a safer, more precise, and thorough cancer operation.
Figure 1
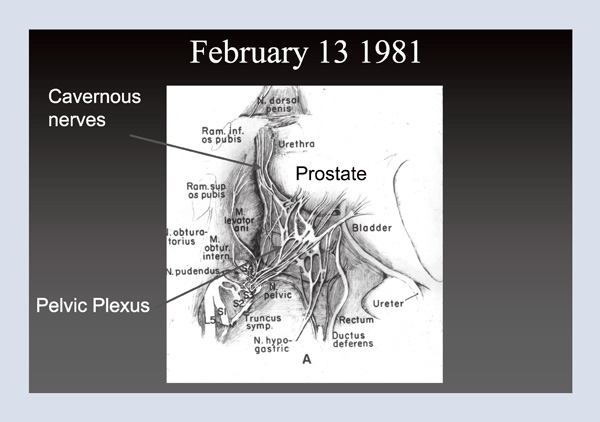
In 1977, soon after the technique for controlling bleeding was developed, a fifty-eight-year- old man returned three months following surgery and told me that he was fully potent. I wondered, how could this be? At that time, everyone believed that because all men were impotent following surgery, the nerves had to run through the prostate. But I knew from this one case that this was not true. So where were the nerves? The answer was not in any textbook. In 1981, I was a visiting professor at the University of Leiden. I spent the afternoon with neuro-urologist Pieter Donker, professor emeritus and former chairman of the department, who was using a dissecting microscope to study (in a stillborn male infant) the nerves that innervate the bladder. When I asked to see the branches to the corpora cavernosa, the nerves responsible for erectile function, he said that he had never looked. Three hours later, we had identified them outside the prostate. Figure 2 shows the prostate, the urethra, and bladder in the infant cadaver. The nerves that we dissected out reveal the branches to the corpora cavernosa, clearly outside the prostate. Based on this observation, we knew where the cavernous nerves were located in a tiny fetus, much the same way you might have a schematic for your television set, but how would you find that transistor? That is, how could we identify these microscopic structures in the adult male pelvis?
Figure 2
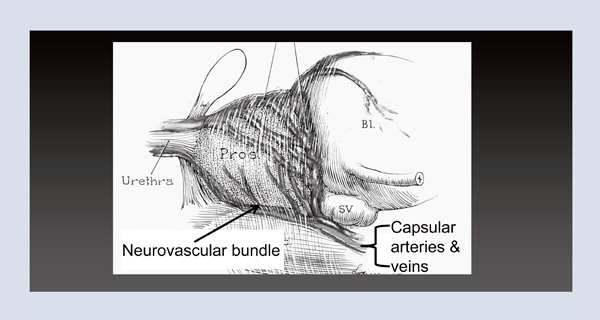
When I returned to Hopkins, I once again used the operating room as an anatomy laboratory. There I identified that the capsular arteries and veins of the prostate traveled in exactly the same location as the nerves in the fetus and speculated that this neurovascular bundle could be used as the intraoperative landmark to identify these microscopic nerves (Figure 3). Armed with that information, on April 26, 1982, I performed the first purposeful nerve-sparing radical prostatectomy. Next month, that patient will have lived thirty years cancer free and with a normal quality of life.
Figure 3
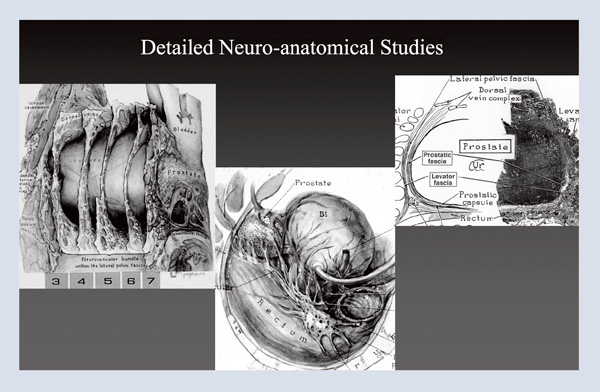
From there, I went on to perform detailed neuroanatomical studies, providing templates for surgeons and delineating the fascia around the prostate (Figure 4). My hope was to make radical prostatectomy a better cancer operation, and I embarked on a twenty-nine-year journey to perfect the technique. Again, using the operating room as an anatomy laboratory, I changed one thing at a time, resulting in twenty-eight major changes over twenty-nine years in 4,569 patients. I maintained a database from day one, documenting changes in technique, cancer control, and quality of life. I constantly reevaluated outcomes, and eventually, I used video for documentation. I found that I could perform the same operation on two men on the same day, and while one of them would be in perfect health at three months, the other would take a year or longer to recover. I learned that minor differences in technique had a major impact, so I videotaped cases, looked at outcomes, reviewed those videotapes frame by frame (yes, my wife is a saint for allowing me to spend my summer vacations doing that) to identify some of the changes that were made. I then used these videos to teach others.
Figure 4
What were the accomplishments of this work? First, there was a major reduction in death from prostate cancer. Prostate cancer is the most common cancer in men. It is also the second most common cause of cancer death in men in the United States. The Scandinavian Prostate Cancer Group carried out a very brave randomized trial, randomizing men to surgery versus watchful waiting, and last year, in The New England Journal of Medicine, the fifteen-year followup study was published.2 Among the men who had benefited the most – that is, men under the age of sixty-five who are most likely to live fifteen years – there was, across the board, a 50 percent relative reduction in metastasis, in death from any cause and in death from cancer.
What is the impact of these results? In 1983, only 7 percent of men with prostate cancer underwent surgery, and radiotherapy was too underpowered to cure. Essentially, no one was being treated with curative intent. However, with the reduction in side effects and improved safety, by 1993, one-third of men – one hundred thousand men that year – underwent surgery. If we apply the results of the Scandinavian trial at fifteen years to today, there should be a dramatic reduction in the number of men dying of the disease or suffering from painful metastasis. Figure 5 shows the changes in mortality in cancers in men and women between 1994 and 2003. The greatest decline in mortality over that decade was the decline in deaths from prostate cancer. The operation was also safer with a reduction in blood loss, which meant that the thirty-day mortality rate fell from 2 percent to 0.2 percent, and the length of stay in the hospital decreased from two weeks to the one to two days that it is today. Another impact is improved quality of life. Today, significant incontinence should be less than 2 percent and sexual function can be preserved in 80 to 90 percent of men who have normal sexual function preoperatively, if it is possible (for optimal cancer control) to preserve both neurovascular bundles and if the procedure is performed by a skilled surgeon.
Figure 5
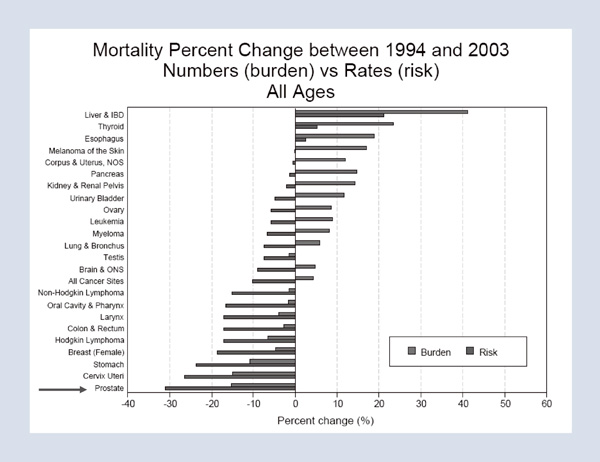
What has been the impact on research? In breast and colon cancer, tissue was always available for pathologic correlation and biochemical molecular study, which accelerated discovery in these fields. However, prior to the development of nerve-sparing radical prostatectomy, only 7 percent of men with localized disease underwent surgery, and thus only small needle biopsy specimens were available for research. However, the availability of tissue harvested from surgical specimens today has galvanized research. In the long run, this impact on research may be the contribution of surgery that may have the greatest impact in reducing deaths from cancer.
In summary, the impact of anatomic discoveries are: improved surgical exposure, reduced blood loss, wider surgical margins, the ability to preserve potency, improved urinary continence, reduction in deaths from prostate cancer, and the availability of tumor tissue, which has galvanized research in the field.
In closing, I would like to thank the residents, faculty, and support staff, past and present, at Hopkins. Over the last thirty years, they have made possible the discoveries that I have summarized in this presentation. I would also like to thank my patients, who have been my partners in discovery.
ENDNOTES
©2013 by David C. Page, Patricia K. Donahoe, and Patrick C. Walsh, respectively
To view or listen to the presentations, visit https://www.amacad.org/content/events/events.aspx?d=479.